The tumour microenvironment presents a “soil” on which cancer cells, “the seeds”, depend on. In the most aggressive and lethal brain tumour glioblastoma, its unique stromal cells composition is including neuronal and glial progenitor cells, neurons, astrocytes, oligodendrocytes, microglia and macrophages, endothelial cells and mesenchymal stem cells. Standard and novel glioblastoma therapies, such as immunotherapies, as well target and alter the tumour microenvironment. The success of cancer treatment thus relies on the comprehension of the highly heterogeneous and immunosuppressive microenvironment to eliminate its tumour supportive role. Despite isolated cancer and stromal cell analyses of cancer and stromal cells that have enabled a better understanding of the glioblastoma microenvironment at the molecular and cellular level, it remains poorly understood due to the lack of appropriate in vitro models.
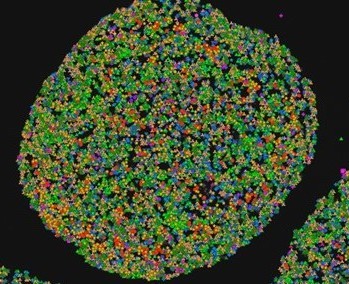
In this project we propose to introduce novel 3D in vitro model of glioblastoma organoid, prepared from patient-derived tumours (Workpackge 1). The organoids that in contrast to 3D glioblastoma cells and stem cells used so far, capture both, glioblastoma and the tumour microenvironment stromal cells. The in vivo tumour intra- and inter-tumour heterogeneity is thus preserved in organoids that are recapitulating histological features of glioblastoma subtypes, as well as capturing the entire diversity of stromal, in particular immune cells. As this model has been only introduced recently, there is a lack of the experimental approaches that would enable to define: first, cell spatial distribution and secondly, their intercellular crosstalk, i.e. communication, comprising of molecular networks that are based on in situ activity among glioblastoma and stromal cells. Next, the organoids will be irradiated and exposed to chemotherapy with temozolomide to mimic standard of-care treatment used in clinics in vivo.
The Workpackage 2 of proposed research project is addressing the visualisation of the gene expression profile in glioblastoma organoids of the most important immune cell components, such as tumour associated macrophages, mesenchymal stem cells, as well as lymphocytes T- cell sand natural killer cells that contribute to glioblastoma resistance to therapy. Their abundance along with their spatial distribution will be revealed in the organoid tissue sections. Taken together non-invasive in situ sequencing method, simultaneously with the subcellular location, prior and post targeted treatment will be used.
Moreover, we will set up the experimental approaches that enable to uncover specific cellular cross talks between chemokine networks, which are based on in situ activity of cancer cells and stromal cells in the tumour microenvironment and are important for glioblastoma response to therapy. In the Workpackage 3, we will focus on chemokine CCL5 and its receptor CCR5 and inhibitor Maraviroc, which we have previously reported to be involved in glioblastoma invasion and reveal how their interaction influences therapeutic effect in the organoids. Secondly, as glioblastoma, immunotherapy has not been as effective as in others solid tumours, we will provide new insight and clues how to increase the efficacy of these approaches by adjuvant immunotherapy. We will model T-cell base immunotherapy treatment on organoids by treatment with PD-1 inhibitor Pembrolizumab, which is used in a clinical trials of immune therapy in glioblastoma. In Workpackage 4 we will develop means/algorithms to link the abundance and composition of immune cells with altered chemokine pathways upon treatment. High resolution of this novel technique allows to reveal novel important changes of cell compositions and of protein interactions that are key biomarkers of tumour-stromal cell crosstalk, upon treatment, and are therefore important for glioblastoma response to therapy.

Experimental workflow of the project.
We are open for collaborations, if you’re interested please contact dr. Metka Novak.